Anionic Polymerization
Anionic polymerization is a form of chain-growth polymerization that encompasses the polymerization of vinyl monomers with strong electronegative groups. This type of polymerization is often employed to produce synthetic polydiene rubbers, solution styrene-butadiene rubbers (SBR), and thermoplastic styrenic elastomers.1,2
The initiation step of an anionic polymerization involves a nucleophilic attack on a monomer resulting in carbanion. Basically, all vinyl monomers with (strong) electronegative substituents polymerize readily in the presence of these anions. Some electron-withdrawing substituents that stabilize the negative charge through charge delocalization, and hence permit stable anionic polymerization include -CN, -COOR, -C6H5, and -CH=CH2, to name only a few. Therefore, monomers such as styrenes, dienes, acrylates and methacrylates, aldehydes, epoxides, acrylonitriles and cyanoacrylates readily undergo anionic polymerization reactions.
The
electron donors (or initiators) are either electron transfer agents
or strong anions. The transfer of an electron from a donor molecule
to the vinyl monomer leads to the formation of an anion radical, the
so-called carbanion:
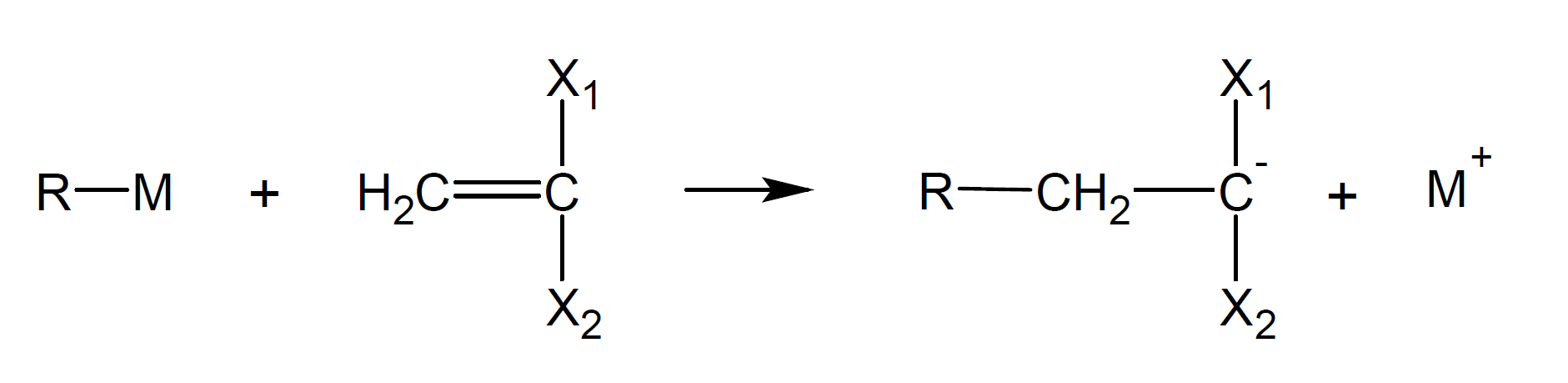
Typical electron donors (Lewis bases or nucleophiles) are alkali metals, such as lithium or sodium. Other strong nucleophilic initiators include covalent or ionic metal amides, alkoxides, hydroxides, amines, phosphines, cyanides, and organometallic compounds such as alkyl lithium compounds and Grignard reagents.1-3 The initiation proceeds either by addition of a neutral (B:) or negative (B:-) nucleophile to the monomer. For example, the initiation and polymerization of styrene with potassium amide proceeds as follows:4
KNH2 ⇔ K+ + NH2-
NH2- + M → NH2M-
NH2Mn- + M → NH2Mn+1-
NH2Mn- + NH3 → NH2MnH + NH2-
The "Gegen" ion, K+, has been omitted from the scheme above, because it is dissolved ("free") in a media of comparatively high dielectric constant.4,5
In carefully controlled systems (pure reactants and inert solvents), an anionic polymerization does not undergo termination reactions. Hence, the chains will remain active indefinitely unless there is deliberate termination or chain transfer. This has two important consequences:
The number average molecular weight, Mn, of the polymer can be calculated from the amount of initiator and amount of consumed monomer, because the degree of polymerization is the ratio of the moles of monomer consumed to the moles of the initiator added: MWn = MW0 [M0] / [I],
where MW0 is the molecular weight of the repeat unit and [M0] and [I] the (initial) molar concentrations of the monomer and the initiator.-
Since all chains are initiated at roughly the same time, the polymer synthesis can be done in a controlled manner. In fact, it is the only one that leads to well defined and nearly mono-disperse molecular weight distribution (Poisson distribution) and structural and compositional uniformity.
This type of polymerization is called living polymerization.
Anionic polymerization can also be used to functionalize polymers, for example by reacting the active chain ends with electrophilic reagents which yields a wide variety of telechelic polymers. The electrophilic reagents (epoxide, aziridine, CO2, etc.) are usually added at the end of the polymerization. End-groups that have been produced in this way include -OH, -SH, -NH2, COCH3, and -COOH, to name only a few.7
An alternative approach for functionalizing polymers is to begin the polymerization with a functional anionic initiator (see example above).
References & Notes
- C.E. Carraher, Seymour/Carraher's Polymer Chemistry, 7th Ed., CRC Press, Boca Raton 2008
- M.D. Lechner, K. Gehrke, E.H. Nordmeier, Makromolekulare Chemie, Birkhauser, Basel 1993
- R.O. Ebewele, Polymer Science and Technology, CRC Press, New York 2000
- Paul L. Flory, Principles of Polymer Chemistry, Ithaca, New york, 1953
The counter ions are only "free" in solvents with comparatively high dielectric constant. For most other cases, the anions may be either tightly associated with the cation or they may be loosely associated with the solvated counter ion.6
- Raymond M. Fuoss, J. Am. Chem. Soc., 80, 19, 5059 - 5061 (1958)
- D.N. Schulz, A.O. Patil, Functional Polymers, ACS Symposium Series; Washington, DC (1998)
Revised July 19, 2020